
a new era in oculometrics
![]()
quantitative pupillometry
![]() ocular alignment
ocular alignment
![]() fusional vergence
fusional vergence
![]() saccades
saccades
![]() smooth pursuit
smooth pursuit
![]() gaze holding
gaze holding
![]() visual field screening
visual field screening
Recent Publications
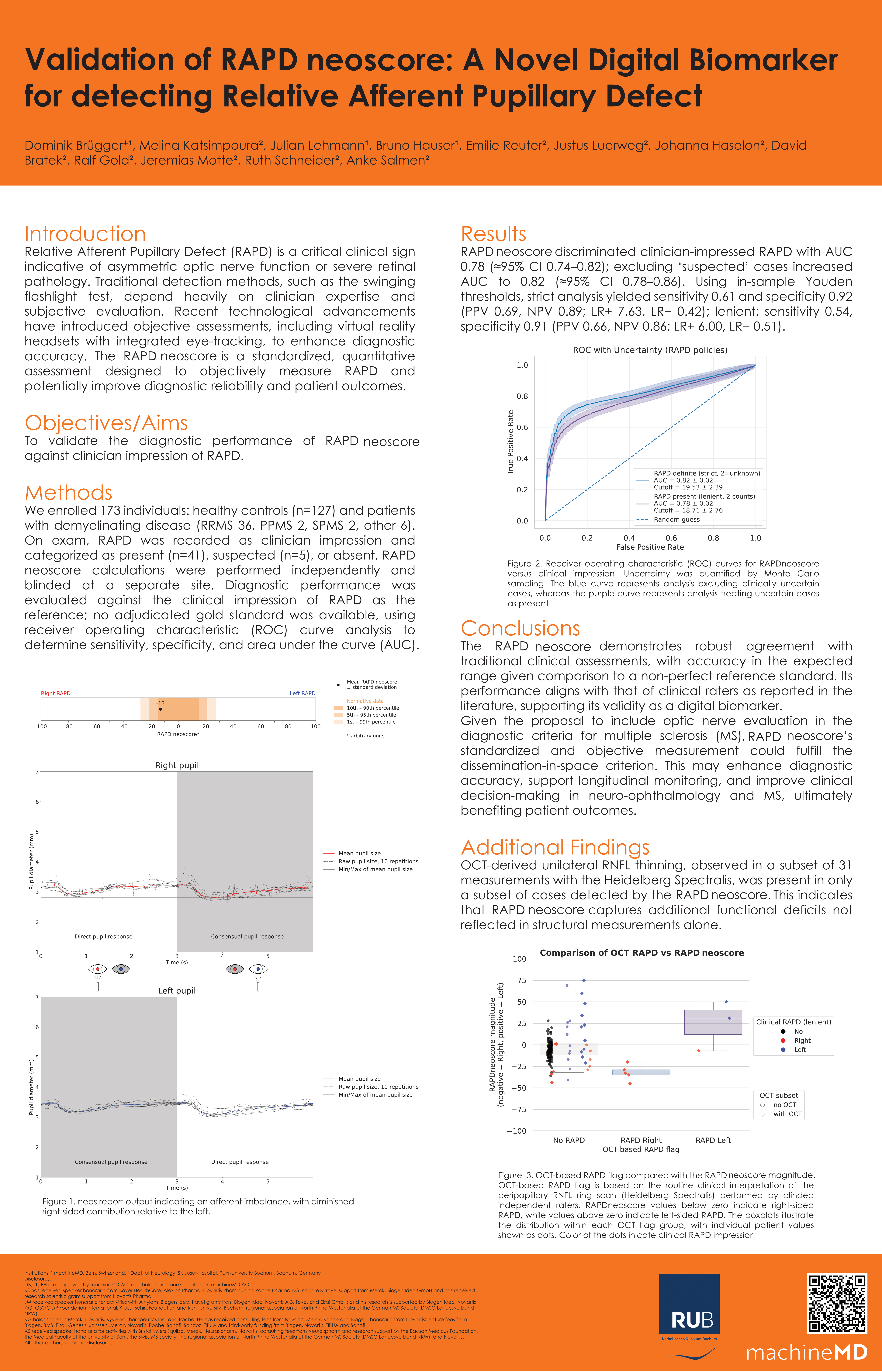
Validation of RAPD neoscore: A Novel Digital Biomarker for detecting Relative Afferent Pupillary Defect (see poster)
Brügger D, Katsimpoura M, Lehmann J, Hauser B, Reuter E, Luerweg J, Haselon J, Bratek D, Motte J, Schneider R, Salmen A.
Multiple Sclerosis Journal, Volume 31, Issue 3_suppl: 41st - 2025
doi: 10.1177/13524585251358344
Case Report: Improvement of diplopia due to severe internuclear ophthalmoplegia by 4-aminopyridine documented using a novel virtual reality-based oculography headset
Reuter E, Luerweg J, Schneider R, Klimas R, Motte J, Gold R, Salmen A.
Frontiers in Virtual Reality, Volume 6 - 2025.
doi: 10.3389/frvir.2025.1595694
Test-retest reliability of gaze precision of a novel virtual reality-based medical device
Coito A, Naidu A, Lehmann J, Hauser B, Brügger D, Abegg M.
Frontiers in Virtual Reality, Vol. 6 - 2025. doi: 10.3389/frvir.2025.1502679
Automated Measurement of Strabismus Angle Using a Commercial Virtual Reality Headset
Rino V, Dominik B, Hilary G, Mathias A.
Klin Monbl Augenheilkd 2024. doi: 10.1055/a-2466-0284
Use of a Novel Virtual Reality-Based Pupillography Device for Glaucoma Management: Cross-Sectional Cohort Pilot Study.
El-Koussy K, Höhn R, Falcão MBL, Hauser B, Naidu A, Abegg M.
Klin Monbl Augenheilkd. 2024 Dec 6. doi: 10.1055/a-2460-0147.
neos® is registered as a Class I medical device in the USA by machineMD AG. Registered Owner Operator Number: 10089071.
Distributed in the USA by machineMD Inc., 1 Broadway, Cambridge, MA 02142.
neos® is a certified Class IIa medical device in accordance with the European Union Medical Device Regulation (EU-2017/745 MDR) by TÜV SÜD Danmark (NB 2443); EUDAMED Actor ID: CH-MF-000041378; Basic UDI-DI: 7649989541-neos-0XX-F5. MHRA Reference Number: 33043. Swiss manufacturer number: CHRN-MF-20003868.
machineMD AG owns patent applications covering aspects of neos® including, but not limited to, WO2023285541A1, WO2023285542A1, WO2025046001A1, WO2025062048A1, and WO2025109221A1.
neos® is a registered trademark of machineMD AG.


